“Computational Fluid Dynamics (CFD) modeling can be used in designing a cleanroom or clean air device by providing information on their likely airflow patterns and allowing the designer to optimize the design and achieve a more effective and efficient installation” [1].
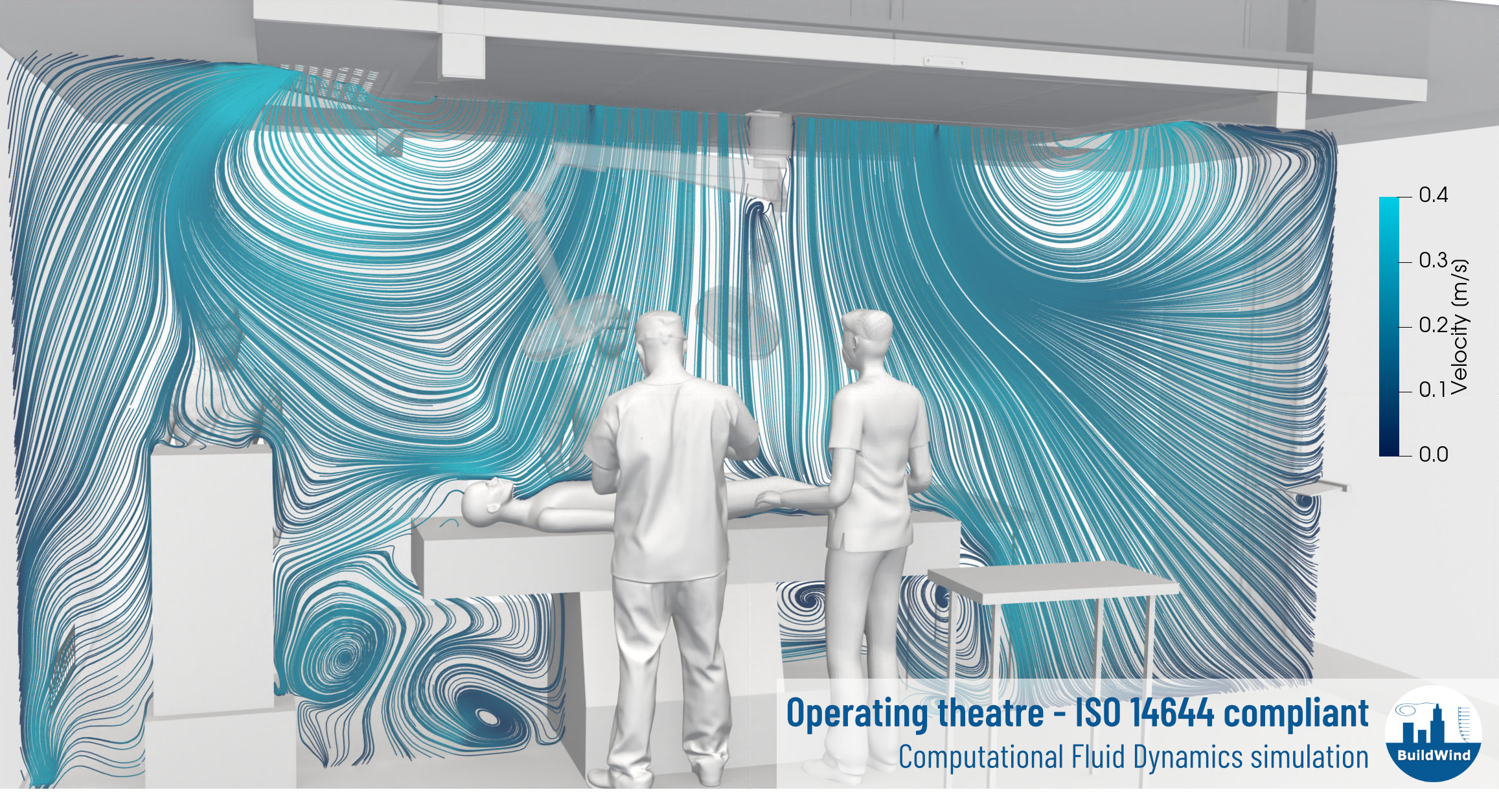
In operating theatres an optimal flow injection by the ventilation system is required to ensure the effective removal of airborne contaminants from the surgical field and to minimize the pressure drops and energy consumption.
CFD can assess the ability of operating theatre ventilation system to
- deliver a laminar flow,
- create a washing effect over the operating table,
- ensure thermal comfort.


Recovery Test - ISO 14644
Cleanliness recovery performance after a particle generation event is one of the most important abilities of an installation” [1].

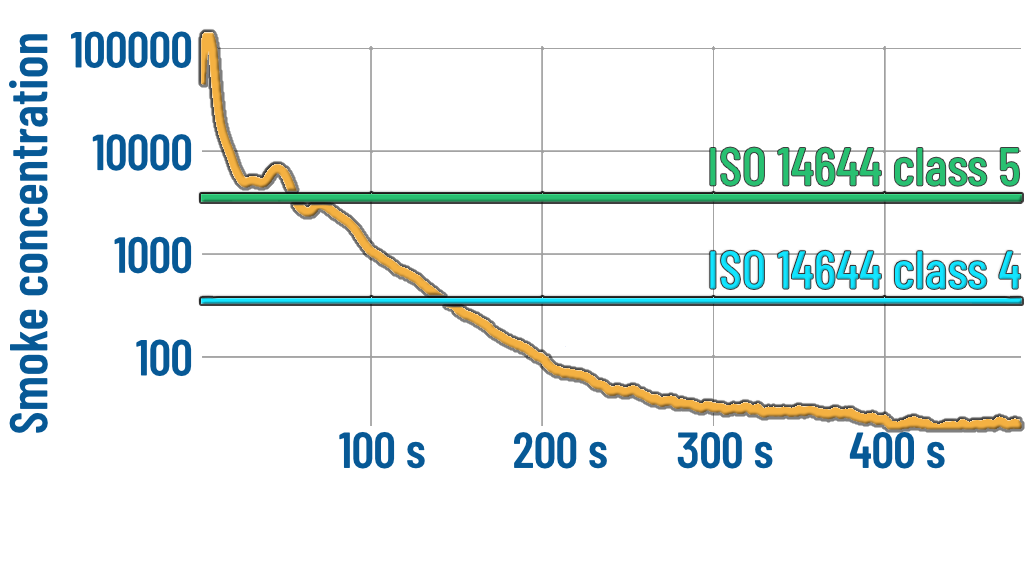
CFD can be used to assess the performance of the operating theatre ventilation system by simulating a recovery test in compliance with ISO 14644.
The recovery test consists in rapidly contaminating the surgical field (clean zone) with smoke, reaching at least a concentration more than 10 times the target cleanliness level [1]. The smoke concentration is then monitored with the ventilation system in operation. The airflow generated by the ventilation system rapidly removes smoke from the clean zone. The recovery performance is then assessed according to ISO 14644 [1] to ensure compliance with recommended guidelines [2].




Once a digital model of the operating theatre and its ventilation system has been developed and validated, different operating theatre scenarios can be accurately simulated with Computational Fluid Dynamics. This provides extremely detailed data of airflow, temperature and contaminant concentration, allowing for a better, faster and cost-effective analysis of the ventilation system performance and its possible improvement.
[1] ISO 14644. “Cleanrooms and associated controlled environments”
[2] EU GGMP. The rules governing medicinal products in the European Union – Volume 4 – EU guidelines to good manufacturing practice – medicinal products for human and veterinary use – Annex 1 – Manufacture of sterile medicinal products. European Commission, Brussels, 2022.

